Our company
A French CRO dedicated to biomedical research
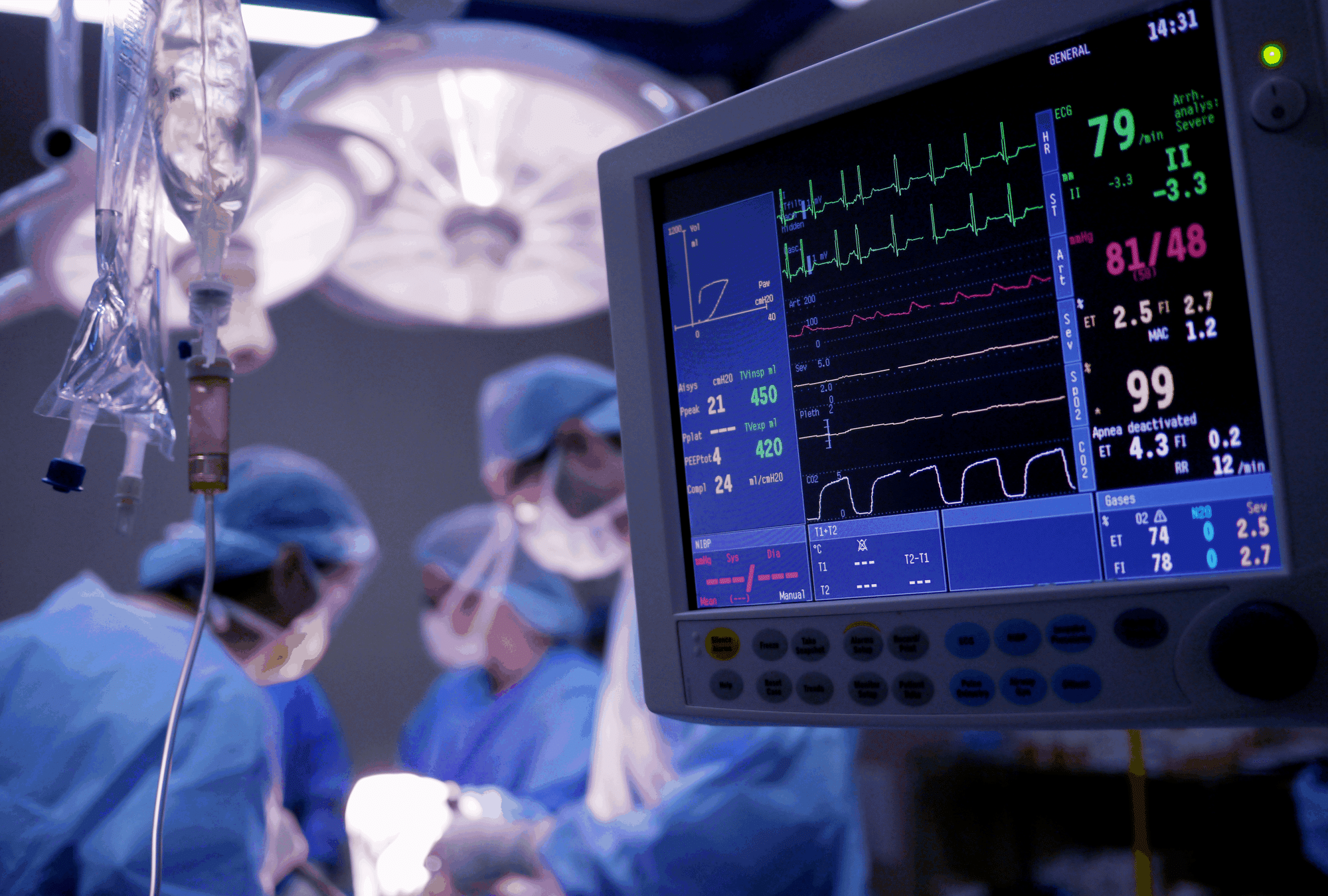
Biossan helps its academic and industrial partners develop their innovations by performing preclinical trials under controlled conditions.
Biossan specializes in evaluating medical devices, new techniques, and pharmaceutical products in healthy and pathological models.
Medical Research
We provide a complete services offer at each step of preclinical research projects
Training of practitioners
We host training workshops and demonstrations for health practitioners